
Inorganic salts vs chelated nutrients Illustration
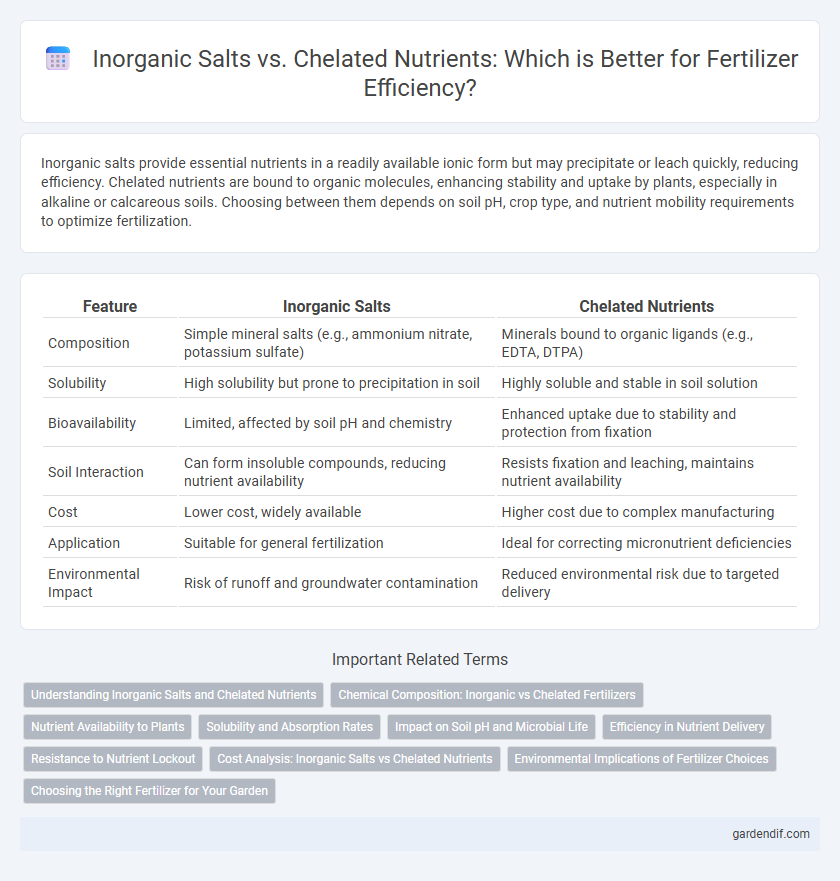
Inorganic salts provide essential nutrients in a readily available ionic form but may precipitate or leach quickly, reducing efficiency. Chelated nutrients are bound to organic molecules, enhancing stability and uptake by plants, especially in alkaline or calcareous soils. Choosing between them depends on soil pH, crop type, and nutrient mobility requirements to optimize fertilization.
Table of Comparison
| Feature | Inorganic Salts | Chelated Nutrients |
|---|---|---|
| Composition | Simple mineral salts (e.g., ammonium nitrate, potassium sulfate) | Minerals bound to organic ligands (e.g., EDTA, DTPA) |
| Solubility | High solubility but prone to precipitation in soil | Highly soluble and stable in soil solution |
| Bioavailability | Limited, affected by soil pH and chemistry | Enhanced uptake due to stability and protection from fixation |
| Soil Interaction | Can form insoluble compounds, reducing nutrient availability | Resists fixation and leaching, maintains nutrient availability |
| Cost | Lower cost, widely available | Higher cost due to complex manufacturing |
| Application | Suitable for general fertilization | Ideal for correcting micronutrient deficiencies |
| Environmental Impact | Risk of runoff and groundwater contamination | Reduced environmental risk due to targeted delivery |
Understanding Inorganic Salts and Chelated Nutrients
Inorganic salts in fertilizers provide essential nutrients in simple ionic forms that are readily available for plant uptake but may quickly leach from the soil. Chelated nutrients consist of mineral ions bound to organic molecules, enhancing nutrient stability and bioavailability by preventing precipitation and immobilization. Understanding the differences between these nutrient forms aids in selecting fertilizers that optimize plant nutrition and minimize environmental impact.
Chemical Composition: Inorganic vs Chelated Fertilizers
Inorganic fertilizers consist primarily of simple mineral salts such as ammonium nitrate, potassium chloride, and superphosphate, providing essential nutrients in readily soluble ionic forms. Chelated fertilizers contain micronutrients bound to organic molecules like EDTA or DTPA, enhancing nutrient stability and availability by preventing precipitation and facilitating controlled release. The chemical composition of chelated nutrients improves plant uptake efficiency compared to the more reactive and sometimes less stable inorganic salts commonly used in traditional fertilizers.
Nutrient Availability to Plants
Inorganic salts provide essential nutrients through rapid solubility, making minerals like potassium chloride and ammonium nitrate readily available to plants for immediate uptake. Chelated nutrients enhance nutrient availability by stabilizing trace elements such as iron, zinc, and manganese, preventing precipitation and fixation in soil, which improves bioavailability. The choice between inorganic salts and chelated nutrients directly impacts nutrient efficiency and plant growth, especially in varying soil pH and composition conditions.
Solubility and Absorption Rates
Inorganic salts, such as ammonium nitrate and potassium sulfate, exhibit high solubility, enabling rapid nutrient release and immediate plant absorption, but they are prone to leaching losses. Chelated nutrients, including iron-EDTA and zinc-DTPA, offer enhanced stability and slower release by preventing precipitation and maintaining availability in various soil pH levels, resulting in more efficient nutrient uptake. The difference in absorption rates is significant, with chelated forms providing sustained nutrient supply that supports prolonged growth compared to the quick but short-lived availability from inorganic salts.
Impact on Soil pH and Microbial Life
Inorganic salts commonly lower soil pH by increasing acidity, potentially harming beneficial microbial populations, whereas chelated nutrients maintain more stable pH levels, supporting healthier microbial diversity. Chelated forms enhance nutrient availability without disrupting soil chemistry, promoting sustainable soil fertility. The balanced pH environment fostered by chelated nutrients improves microbial activity critical for nutrient cycling and organic matter decomposition.
Efficiency in Nutrient Delivery
Inorganic salts provide nutrients in simple ionic forms, allowing rapid uptake but are prone to leaching and volatilization, reducing efficiency over time. Chelated nutrients feature a stable organic ligand complex, enhancing nutrient availability and preventing precipitation in soils with high pH or heavy metal presence. This increases nutrient delivery efficiency, especially for micronutrients like iron, zinc, and manganese, crucial for optimal plant growth.
Resistance to Nutrient Lockout
Inorganic salts often face challenges with nutrient lockout due to their tendency to react and precipitate in the soil, reducing nutrient availability to plants. Chelated nutrients maintain higher bioavailability by protecting essential micronutrients from soil interactions, enhancing resistance to nutrient lockout. This efficient nutrient delivery supports consistent plant uptake and improved growth outcomes.
Cost Analysis: Inorganic Salts vs Chelated Nutrients
Inorganic salts generally offer a lower upfront cost compared to chelated nutrients, making them more attractive for large-scale agricultural applications with tight budgets. Chelated nutrients, while more expensive initially, provide improved nutrient bioavailability and reduced precipitation losses, potentially lowering overall nutrient use and long-term costs. A comprehensive cost analysis must weigh the immediate price difference against enhanced efficiency and crop yield benefits associated with chelated formulations.
Environmental Implications of Fertilizer Choices
Inorganic salts commonly used in fertilizers such as ammonium nitrate and superphosphate often lead to nutrient runoff, causing eutrophication and water quality degradation in aquatic ecosystems. Chelated nutrients, particularly those involving micronutrients like iron, zinc, and manganese, improve nutrient uptake efficiency and reduce leaching risks, minimizing environmental contamination. Selecting chelated forms over conventional inorganic salts contributes to sustainable agriculture by lowering the ecological footprint and preserving soil and water health.
Choosing the Right Fertilizer for Your Garden
Inorganic salts offer readily available nutrients like nitrogen, phosphorus, and potassium that rapidly boost plant growth but may lead to nutrient leaching and soil imbalance. Chelated nutrients, such as iron chelates and zinc chelates, enhance micronutrient stability and absorption efficiency, especially in alkaline or calcareous soils. Selecting the right fertilizer involves assessing soil pH, nutrient availability, and plant needs to balance quick nutrient release and sustained uptake for optimal garden health.
Inorganic salts vs chelated nutrients Infographic
 gardendif.com
gardendif.com