
Urea vs ammonium sulfate Illustration
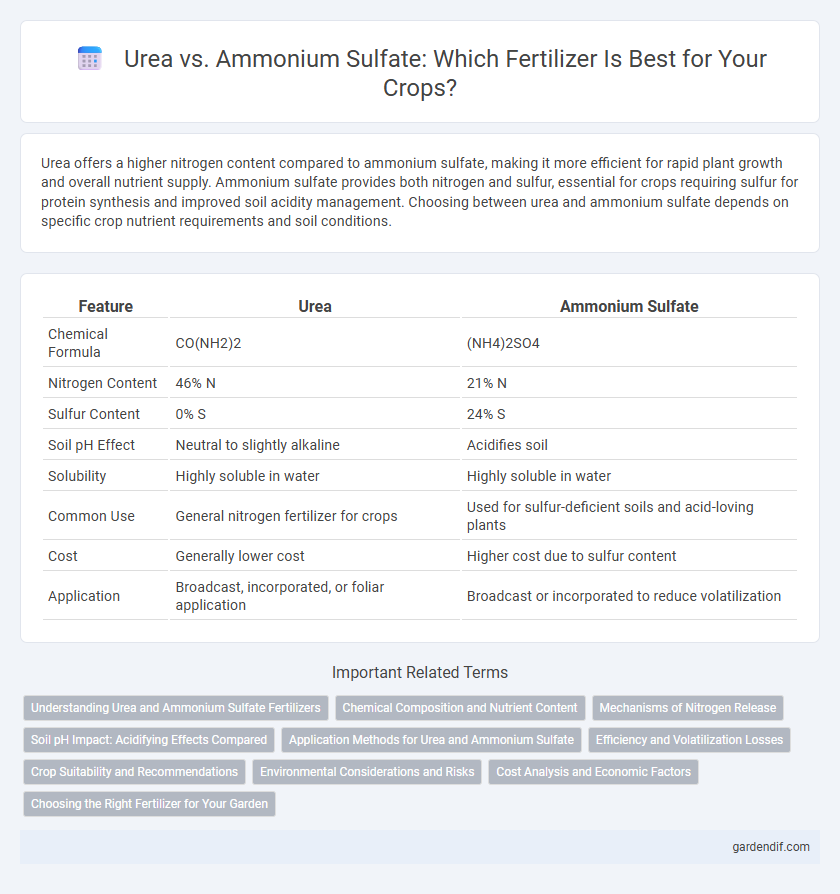
Urea offers a higher nitrogen content compared to ammonium sulfate, making it more efficient for rapid plant growth and overall nutrient supply. Ammonium sulfate provides both nitrogen and sulfur, essential for crops requiring sulfur for protein synthesis and improved soil acidity management. Choosing between urea and ammonium sulfate depends on specific crop nutrient requirements and soil conditions.
Table of Comparison
| Feature | Urea | Ammonium Sulfate |
|---|---|---|
| Chemical Formula | CO(NH2)2 | (NH4)2SO4 |
| Nitrogen Content | 46% N | 21% N |
| Sulfur Content | 0% S | 24% S |
| Soil pH Effect | Neutral to slightly alkaline | Acidifies soil |
| Solubility | Highly soluble in water | Highly soluble in water |
| Common Use | General nitrogen fertilizer for crops | Used for sulfur-deficient soils and acid-loving plants |
| Cost | Generally lower cost | Higher cost due to sulfur content |
| Application | Broadcast, incorporated, or foliar application | Broadcast or incorporated to reduce volatilization |
Understanding Urea and Ammonium Sulfate Fertilizers
Urea is a high-nitrogen fertilizer containing 46% nitrogen, making it one of the most concentrated nitrogen sources for crop nutrition. Ammonium sulfate provides 21% nitrogen and 24% sulfur, supplying essential nutrients that support protein synthesis and improve soil fertility. Understanding the nitrogen release patterns and sulfur content differences between urea and ammonium sulfate helps optimize fertilizer selection based on soil nutrient needs and crop requirements.
Chemical Composition and Nutrient Content
Urea contains about 46% nitrogen in a highly concentrated form, making it one of the most nitrogen-rich fertilizers available. Ammonium sulfate provides approximately 21% nitrogen along with 24% sulfur, essential for plant protein synthesis and enzyme function. The chemical composition of urea is CO(NH2)2, while ammonium sulfate is (NH4)2SO4, reflecting their distinct nutrient contributions and soil interaction characteristics.
Mechanisms of Nitrogen Release
Urea releases nitrogen through hydrolysis, converting into ammonium ions that plants readily absorb, making it a fast-acting fertilizer with high nitrogen content (46%). Ammonium sulfate provides nitrogen in both ammonium and sulfate forms, offering a slower release due to soil microbes converting ammonium to nitrate, enhancing nitrogen availability over time while improving soil sulfur levels. The choice between urea and ammonium sulfate depends on crop nitrogen demand timing and soil sulfur requirements, influencing nitrogen use efficiency and soil health.
Soil pH Impact: Acidifying Effects Compared
Urea and ammonium sulfate both impact soil pH, but ammonium sulfate has a stronger acidifying effect due to its higher ammonium content and sulfate ions, which lower soil pH more rapidly. Urea initially raises pH slightly during hydrolysis but eventually contributes to acidification as ammonium converts to nitrate through nitrification. Selecting between these fertilizers depends on soil buffering capacity and crop sensitivity to pH changes, with ammonium sulfate preferred for alkaline soils requiring swift acidification.
Application Methods for Urea and Ammonium Sulfate
Urea is commonly applied through broadcasting, fertigation, or foliar spraying, making it versatile for various cropping systems and improving nitrogen use efficiency when incorporated into the soil. Ammonium sulfate is typically applied as a soil amendment through broadcasting or banding, effectively providing both nitrogen and sulfur directly to the root zone. Proper application timing and method for each fertilizer enhance nutrient uptake, reduce volatilization losses, and support optimal crop growth.
Efficiency and Volatilization Losses
Urea typically exhibits higher nitrogen efficiency compared to ammonium sulfate due to its rapid nitrogen release, but it is more susceptible to volatilization losses, especially under alkaline soil conditions without proper incorporation. Ammonium sulfate provides a more stable nitrogen form, reducing volatilization risks and improving nitrogen use efficiency in acidic soils. Optimizing fertilizer choice according to soil pH and application methods significantly minimizes nitrogen loss and enhances crop uptake.
Crop Suitability and Recommendations
Urea provides a high nitrogen content of 46%, making it ideal for nitrogen-demanding crops like wheat and corn, especially in neutral to alkaline soils where rapid nitrogen availability is beneficial. Ammonium sulfate contains 21% nitrogen and 24% sulfur, making it preferred for sulfur-deficient soils and crops such as cotton, rice, and legumes that require both nitrogen and sulfur for optimal growth. For acidic soils, ammonium sulfate improves nitrogen uptake efficiency by lowering soil pH, while urea is recommended for well-drained, non-acidic soils to maximize nitrogen use efficiency.
Environmental Considerations and Risks
Urea and ammonium sulfate differ significantly in their environmental impacts, with urea releasing ammonia gas that can contribute to air pollution and volatilization losses, while ammonium sulfate poses risks of soil acidification due to its sulfate content. Urea's high nitrogen content promotes rapid plant growth but may increase nitrous oxide emissions, a potent greenhouse gas, whereas ammonium sulfate's slower nitrogen release reduces volatilization but can lead to long-term soil fertility decline if misused. Careful management and application timing are crucial to minimizing nitrogen leaching, runoff, and overall environmental risks associated with both fertilizers.
Cost Analysis and Economic Factors
Urea typically offers a lower cost per unit of nitrogen compared to ammonium sulfate, making it a more economical choice for large-scale agricultural applications. However, ammonium sulfate provides additional sulfur, which can reduce the need for separate sulfur fertilization and influence overall cost-effectiveness depending on soil nutrient levels. Economic factors such as local pricing, application rates, and crop nutrient requirements ultimately determine the most cost-efficient fertilizer option between urea and ammonium sulfate.
Choosing the Right Fertilizer for Your Garden
Urea contains 46% nitrogen, making it a highly concentrated nitrogen source ideal for fast-growing plants requiring rapid nitrogen uptake. Ammonium sulfate supplies 21% nitrogen along with sulfur, which improves soil acidity and benefits sulfur-deficient soils. Selecting between urea and ammonium sulfate depends on your garden's soil pH and nutrient needs, with urea suited for neutral to alkaline soils and ammonium sulfate preferred for acidic or sulfur-deficient conditions.
Urea vs ammonium sulfate Infographic
 gardendif.com
gardendif.com