
Urea vs Ammonium nitrate Illustration
Urea contains a higher percentage of nitrogen (46%) compared to ammonium nitrate (33-34%), making it more efficient for nitrogen supply in fertilizers. Urea is less prone to leaching but requires proper management to prevent volatilization losses, whereas ammonium nitrate provides a readily available nitrogen form but poses handling and storage hazards due to its explosive nature. Choosing between urea and ammonium nitrate depends on crop requirements, soil conditions, and safety considerations in fertilizer application.
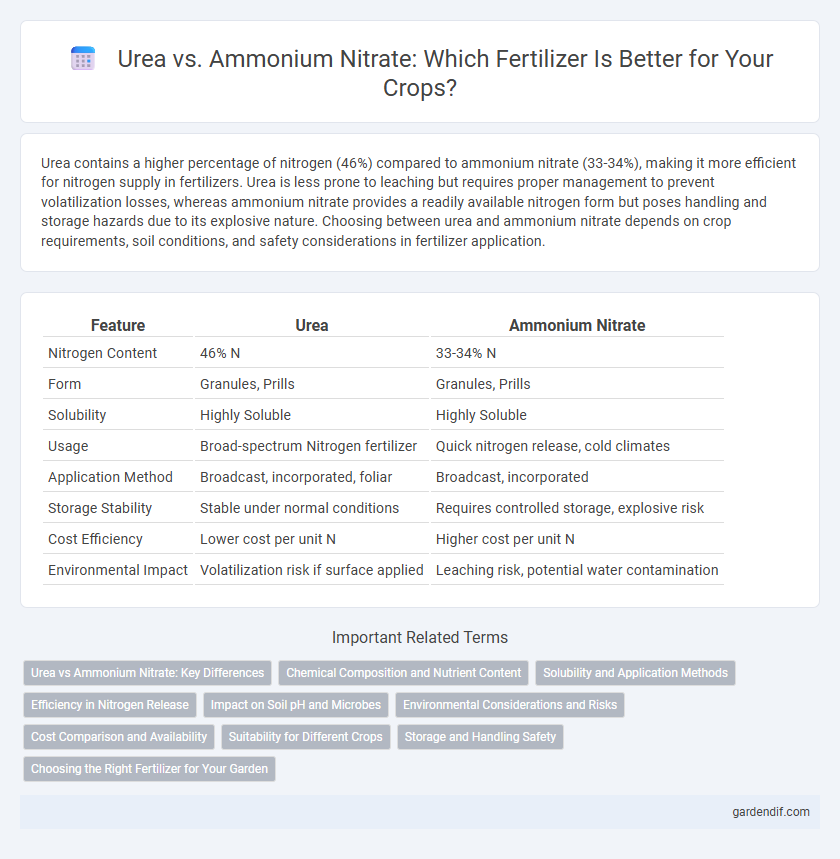
Table of Comparison
| Feature | Urea | Ammonium Nitrate |
|---|---|---|
| Nitrogen Content | 46% N | 33-34% N |
| Form | Granules, Prills | Granules, Prills |
| Solubility | Highly Soluble | Highly Soluble |
| Usage | Broad-spectrum Nitrogen fertilizer | Quick nitrogen release, cold climates |
| Application Method | Broadcast, incorporated, foliar | Broadcast, incorporated |
| Storage Stability | Stable under normal conditions | Requires controlled storage, explosive risk |
| Cost Efficiency | Lower cost per unit N | Higher cost per unit N |
| Environmental Impact | Volatilization risk if surface applied | Leaching risk, potential water contamination |
Urea vs Ammonium Nitrate: Key Differences
Urea contains 46% nitrogen, making it the highest nitrogen content fertilizer compared to ammonium nitrate, which contains approximately 34% nitrogen. Urea is less hygroscopic and safer to store, whereas ammonium nitrate is highly hygroscopic and poses explosion risks, requiring stringent handling protocols. The slower nitrogen release from urea benefits crops with longer growth cycles, while ammonium nitrate's rapid nitrogen availability suits crops needing immediate nutrient uptake.
Chemical Composition and Nutrient Content
Urea contains 46% nitrogen in the form of amide groups, making it the highest nitrogen concentration fertilizer available, while ammonium nitrate provides approximately 33-34% nitrogen split between ammonium and nitrate forms, offering immediate and sustained nitrogen availability. The chemical composition of urea (CO(NH2)2) allows for rapid nitrogen release upon hydrolysis by soil enzymes, whereas ammonium nitrate (NH4NO3) delivers nitrogen directly in two forms that plants can instantly absorb. Nutrient content differences influence their suitability for various crops and soil conditions, with ammonium nitrate often preferred for quick nitrogen uptake and urea favored for its higher nitrogen concentration and cost-effectiveness.
Solubility and Application Methods
Urea exhibits high solubility in water, making it ideal for foliar feeding and fertigation, while ammonium nitrate also dissolves easily but provides both ammonium and nitrate nitrogen forms, supporting rapid nutrient uptake in soil applications. Urea's solubility at 1080 g/L at 20degC enables efficient distribution through drip irrigation systems, whereas ammonium nitrate's solubility of 1180 g/L supports effective use in both soil incorporation and top dressing. Selecting between urea and ammonium nitrate depends on crop nitrogen needs, irrigation practices, and desired release rates.
Efficiency in Nitrogen Release
Urea releases nitrogen through hydrolysis, providing a rapid but sometimes uneven nitrogen supply that may lead to volatilization losses if not incorporated properly. Ammonium nitrate offers a more balanced and immediate nitrogen availability with reduced volatilization risk, enhancing plant uptake efficiency. The choice between urea and ammonium nitrate depends on crop type, soil conditions, and environmental factors impacting nitrogen use efficiency.
Impact on Soil pH and Microbes
Urea application temporarily increases soil pH as it hydrolyzes to ammonia, which can raise pH levels in the root zone, promoting microbial activity that favors nitrogen mineralization. In contrast, ammonium nitrate tends to acidify the soil over time due to nitrification, leading to lower pH and potential suppression of beneficial microbial communities involved in organic matter decomposition. Understanding these pH-driven microbial impacts is vital for optimizing soil health and nutrient cycling when selecting between urea and ammonium nitrate fertilizers.
Environmental Considerations and Risks
Urea releases ammonia into the atmosphere, contributing to air pollution and greenhouse gas emissions, while its runoff can lead to water eutrophication. Ammonium nitrate poses a significant explosion risk during storage and transport, requiring stringent safety measures. Both fertilizers can contaminate water sources, but ammonium nitrate's high solubility increases leaching potential, impacting soil and aquatic ecosystems.
Cost Comparison and Availability
Urea generally offers a lower cost per nitrogen unit compared to ammonium nitrate, making it a more budget-friendly option for large-scale agricultural use. Ammonium nitrate, while more expensive, provides quicker nitrogen release, which can justify its higher price in specific crop cycles. Urea is widely available globally, whereas ammonium nitrate's availability can be restricted due to regulatory controls and safety concerns.
Suitability for Different Crops
Urea is highly suitable for crops like wheat, rice, and maize due to its high nitrogen content and slow nitrogen release, promoting steady growth. Ammonium nitrate is preferred for crops requiring immediate nitrogen availability, such as vegetables and leafy greens, due to its rapid solubility and fast nutrient absorption. Crop-specific factors like soil pH, moisture levels, and nitrogen uptake rates influence the choice between urea and ammonium nitrate for optimal fertilizer efficiency.
Storage and Handling Safety
Urea offers safer storage and handling characteristics compared to ammonium nitrate due to its lower hygroscopicity and non-explosive nature. Ammonium nitrate requires stringent regulations to prevent moisture absorption and avoid risks of accidental detonation under improper conditions. Proper ventilation, temperature control, and segregation from incompatible materials are critical for safe ammonium nitrate storage, while urea poses fewer hazards.
Choosing the Right Fertilizer for Your Garden
Urea contains 46% nitrogen, making it a highly concentrated choice that promotes rapid plant growth, especially in nitrogen-deficient soils. Ammonium nitrate offers a balanced nitrogen release, with approximately 33% nitrogen content, enhancing both immediate and sustained nutrient availability for healthier garden plants. Selecting between urea and ammonium nitrate depends on your soil's nitrogen needs, plant type, and environmental conditions to optimize growth and minimize nutrient loss.
Urea vs Ammonium nitrate Infographic
 gardendif.com
gardendif.com