
Urea fertilizer vs Ammonium nitrate Illustration
Urea fertilizer contains 46% nitrogen, making it a highly concentrated and cost-effective option for promoting rapid plant growth compared to ammonium nitrate, which offers about 34% nitrogen content. Urea is less prone to leaching and volatilization, providing longer-lasting nitrogen availability in the soil, while ammonium nitrate delivers quick nitrogen release but poses higher risks for environmental runoff. Farmers often prefer urea for its efficiency and lower environmental impact, although ammonium nitrate is favored in situations requiring immediate nitrogen availability.
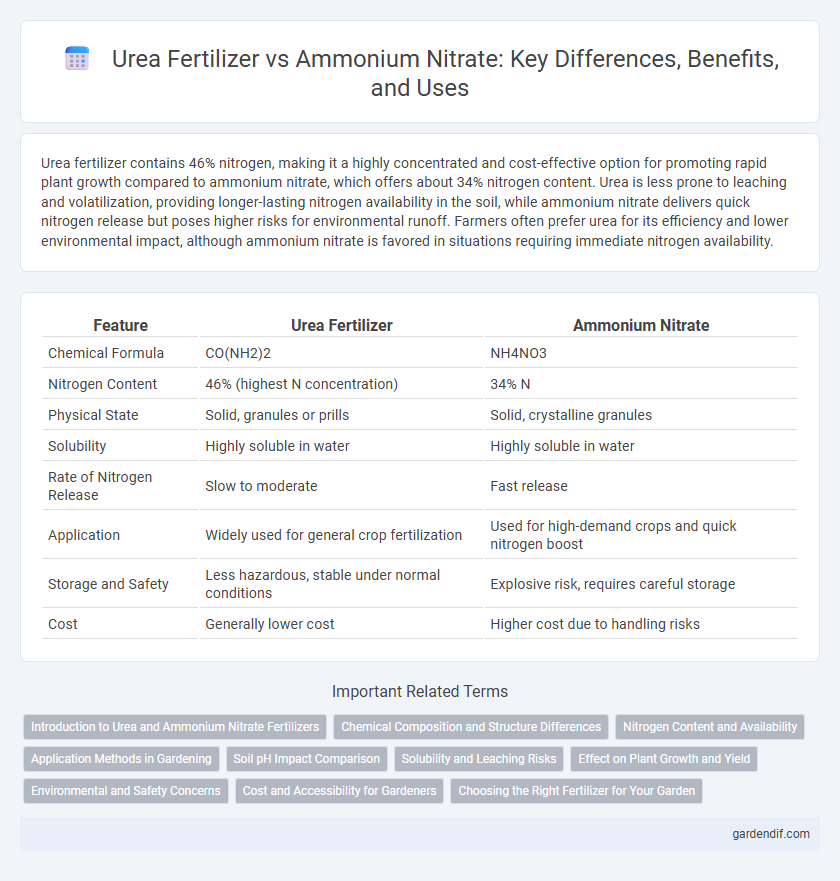
Table of Comparison
| Feature | Urea Fertilizer | Ammonium Nitrate |
|---|---|---|
| Chemical Formula | CO(NH2)2 | NH4NO3 |
| Nitrogen Content | 46% (highest N concentration) | 34% N |
| Physical State | Solid, granules or prills | Solid, crystalline granules |
| Solubility | Highly soluble in water | Highly soluble in water |
| Rate of Nitrogen Release | Slow to moderate | Fast release |
| Application | Widely used for general crop fertilization | Used for high-demand crops and quick nitrogen boost |
| Storage and Safety | Less hazardous, stable under normal conditions | Explosive risk, requires careful storage |
| Cost | Generally lower cost | Higher cost due to handling risks |
Introduction to Urea and Ammonium Nitrate Fertilizers
Urea fertilizer contains 46% nitrogen, making it the highest nitrogen content solid fertilizer widely used for various crops due to its cost-effectiveness and ease of application. Ammonium nitrate fertilizer provides 33-34% nitrogen and is valued for its rapid nitrogen release and high solubility, which supports immediate nutrient availability for plants. Both fertilizers play critical roles in enhancing soil fertility and crop yields but differ in nitrogen content, volatility, and suitability for specific agricultural conditions.
Chemical Composition and Structure Differences
Urea fertilizer contains 46% nitrogen in the form of amide nitrogen (CO(NH2)2), whereas ammonium nitrate provides 33-34% nitrogen by combining ammonium (NH4+) and nitrate (NO3-) ions. Urea is a neutral, organic compound with a molecular structure featuring a carbonyl group bonded to two amine groups, promoting rapid nitrogen release through microbial hydrolysis. Ammonium nitrate is an inorganic salt with an ionic lattice structure, enabling immediate nitrogen availability through dissociation into ammonium and nitrate ions in soil.
Nitrogen Content and Availability
Urea fertilizer contains approximately 46% nitrogen, offering one of the highest nitrogen concentrations among common fertilizers, which promotes rapid plant growth. Ammonium nitrate typically provides around 33-34% nitrogen but has a dual form, supplying both ammonium and nitrate nitrogen, making it readily available for immediate and sustained uptake by plants. The high nitrogen content in urea requires enzymatic conversion to ammonium before plant absorption, whereas ammonium nitrate's nitrogen forms are directly accessible, influencing nutrient availability and efficiency in various soil conditions.
Application Methods in Gardening
Urea fertilizer is typically applied by broadcasting or side-dressing around plants, where its high nitrogen content promotes rapid leaf growth in garden vegetables and flowers. Ammonium nitrate, favored for its faster nitrogen release, is often used in granular form and incorporated directly into the soil to minimize nitrogen loss and ensure consistent nutrient availability. Both fertilizers benefit from precise application timing based on plant growth stages, with urea requiring careful watering to prevent volatilization and ammonium nitrate demanding secure storage due to its reactive properties.
Soil pH Impact Comparison
Urea fertilizer increases soil pH temporarily during hydrolysis, releasing ammonia which can raise alkalinity, whereas ammonium nitrate typically lowers soil pH due to nitrification producing nitric acid, increasing soil acidity. The pH impact of urea is often short-lived and depends on the rate of ammonia volatilization and soil buffering capacity, while ammonium nitrate causes more sustained acidification, potentially affecting nutrient availability. Managing soil pH with lime or other amendments is crucial when using ammonium nitrate to prevent acidification and maintain optimal crop growth conditions.
Solubility and Leaching Risks
Urea fertilizer exhibits high solubility in water, enabling rapid nitrogen availability but increases the risk of leaching in sandy soils due to its conversion to nitrate. Ammonium nitrate also dissolves readily, providing a balanced source of ammonium and nitrate nitrogen, yet its leaching potential can be moderated by soil type and moisture conditions. Effective management practices are essential to minimize nitrogen loss and environmental impact for both fertilizers.
Effect on Plant Growth and Yield
Urea fertilizer supplies a high concentration of nitrogen, promoting rapid vegetative growth and enhancing leaf development, which directly increases crop yield potential. Ammonium nitrate provides both ammonium and nitrate nitrogen forms, facilitating quicker nitrogen uptake and improving overall plant growth efficiency under various soil conditions. Studies show that while urea is cost-effective and widely used, ammonium nitrate often results in higher grain yields due to its balanced nitrogen availability and reduced volatilization losses.
Environmental and Safety Concerns
Urea fertilizer releases ammonia into the atmosphere, contributing to air pollution and posing volatilization risks, while ammonium nitrate is highly hygroscopic and can absorb moisture, leading to caking and handling difficulties. Ammonium nitrate poses significant safety risks due to its explosive potential, requiring strict storage regulations to prevent accidents, whereas urea presents lower explosive hazards but can lead to soil acidification and nitrate leaching. Both fertilizers contribute to environmental issues such as groundwater contamination and greenhouse gas emissions, demanding careful management to minimize ecological and health impacts.
Cost and Accessibility for Gardeners
Urea fertilizer offers a lower cost per pound of nitrogen, making it more budget-friendly for gardeners, while ammonium nitrate tends to be more expensive due to stricter regulations and handling requirements. Urea is widely accessible in most garden centers and agricultural supply stores, whereas ammonium nitrate availability is often limited by legal restrictions and storage regulations. Gardeners seeking an economical and easily obtainable nitrogen source typically prefer urea for small-scale applications.
Choosing the Right Fertilizer for Your Garden
Urea fertilizer contains 46% nitrogen and is favored for its high nitrogen content and cost-effectiveness, promoting rapid plant growth and green foliage. Ammonium nitrate provides 33-34% nitrogen with a balanced ammonium and nitrate form, ensuring quick nutrient availability and sustained nitrogen release for diverse soil types. Selecting the right fertilizer depends on soil pH, crop requirements, and environmental conditions to optimize nitrogen efficiency and minimize nutrient loss.
Urea fertilizer vs Ammonium nitrate Infographic
 gardendif.com
gardendif.com