
pH adjustment vs Soil buffering Illustration
Soil pH adjustment involves applying lime or sulfur to modify acidity or alkalinity, directly influencing nutrient availability for plants. Soil buffering capacity refers to the soil's natural ability to resist pH changes due to its composition and organic matter content. Understanding soil buffering is essential for effective pH management, ensuring long-term soil health and optimal crop growth.
Table of Comparison
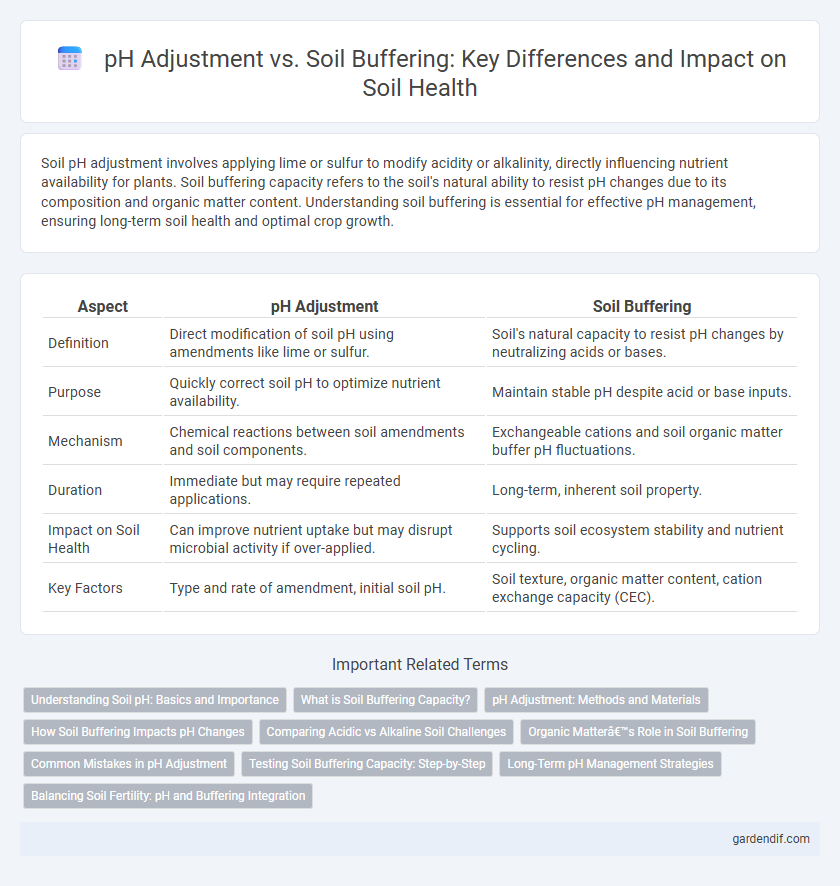
| Aspect | pH Adjustment | Soil Buffering |
|---|---|---|
| Definition | Direct modification of soil pH using amendments like lime or sulfur. | Soil's natural capacity to resist pH changes by neutralizing acids or bases. |
| Purpose | Quickly correct soil pH to optimize nutrient availability. | Maintain stable pH despite acid or base inputs. |
| Mechanism | Chemical reactions between soil amendments and soil components. | Exchangeable cations and soil organic matter buffer pH fluctuations. |
| Duration | Immediate but may require repeated applications. | Long-term, inherent soil property. |
| Impact on Soil Health | Can improve nutrient uptake but may disrupt microbial activity if over-applied. | Supports soil ecosystem stability and nutrient cycling. |
| Key Factors | Type and rate of amendment, initial soil pH. | Soil texture, organic matter content, cation exchange capacity (CEC). |
Understanding Soil pH: Basics and Importance
Soil pH affects nutrient availability and microbial activity, making accurate pH adjustment essential for optimal plant growth. Soil buffering capacity, determined by organic matter, clay content, and cation exchange capacity, influences how much amendment is needed to change pH levels. Understanding the interaction between soil pH and buffering helps in applying lime or sulfur effectively to maintain balanced soil chemistry.
What is Soil Buffering Capacity?
Soil buffering capacity refers to the soil's ability to resist changes in pH when acids or bases are added, maintaining a stable environment for plant growth. This capacity depends on factors such as organic matter content, clay minerals, and cation exchange capacity (CEC), which together neutralize acids or bases to prevent drastic pH fluctuations. Understanding soil buffering capacity is crucial for effective pH adjustment strategies in agriculture and horticulture to optimize nutrient availability and crop yields.
pH Adjustment: Methods and Materials
Soil pH adjustment involves using materials such as lime (calcium carbonate) to raise pH or sulfur compounds to lower pH, effectively modifying soil acidity or alkalinity for optimal plant growth. The choice of amendment depends on soil buffering capacity, which influences the quantity and effectiveness of the material needed for sustained pH change. Accurate soil testing guides the precise application of pH-altering materials, ensuring balanced nutrient availability and improved crop yield.
How Soil Buffering Impacts pH Changes
Soil buffering capacity determines the soil's ability to resist pH changes when lime or acid is applied, controlling nutrient availability and microbial activity. High buffering capacity soils, often rich in clay and organic matter, require larger amendments to shift pH effectively compared to sandy soils with low buffering. Understanding soil buffering mechanisms is essential for precise pH management to optimize crop growth and soil health.
Comparing Acidic vs Alkaline Soil Challenges
Acidic soils often require lime application to raise pH and improve nutrient availability, while alkaline soils demand sulfur treatments to lower pH and enhance metal solubility. Soil buffering capacity, influenced by clay and organic matter content, determines how resistant the soil is to pH changes during these adjustments. Comparing challenges, acidic soils frequently face aluminum toxicity, whereas alkaline soils struggle with nutrient deficiencies like iron chlorosis.
Organic Matter’s Role in Soil Buffering
Organic matter plays a crucial role in soil buffering by stabilizing pH levels through cation exchange capacity and the release of organic acids. It enhances the soil's ability to resist drastic pH changes by binding hydrogen and aluminum ions, thus maintaining nutrient availability and microbial activity. Soils rich in organic matter demonstrate improved resilience against acidification or alkalization compared to mineral soils with low buffering capacity.
Common Mistakes in pH Adjustment
Common mistakes in pH adjustment often stem from neglecting soil buffering capacity, which can lead to over-application of lime or acid and unstable pH levels. Many growers fail to test soil buffering properties, causing sudden swings in pH that harm nutrient availability and microbial activity. Understanding the soil's cation exchange capacity (CEC) and organic matter content is crucial for effective pH management and avoiding costly remediation.
Testing Soil Buffering Capacity: Step-by-Step
Testing soil buffering capacity involves collecting a representative soil sample and preparing it for analysis by air-drying and sieving to remove debris. The sample is then mixed with a known concentration of acid or base, and the pH change is measured after equilibrium is reached, indicating the soil's resistance to pH alteration. Accurate soil buffering capacity testing helps determine lime or sulfur requirements for effective pH adjustment, optimizing nutrient availability and crop growth.
Long-Term pH Management Strategies
Effective long-term pH management strategies prioritize soil buffering capacity to maintain stable nutrient availability and minimize harmful fluctuations caused by acidifying or alkalizing inputs. Regular soil testing combined with the judicious application of lime or sulfur ensures pH levels remain within optimal ranges tailored to specific crop requirements. Incorporating organic matter enhances buffering capacity by improving cation exchange capacity and microbial activity, fostering a resilient soil environment against pH shifts.
Balancing Soil Fertility: pH and Buffering Integration
Balancing soil fertility requires precise pH adjustment considering the soil's buffering capacity, which regulates how resistant soil is to pH changes. Soils with high buffering capacity, rich in organic matter and clay minerals, need more lime or sulfur amendments to shift pH effectively. Understanding the interplay between pH adjustment and soil buffering ensures optimal nutrient availability and sustainable crop production.
pH adjustment vs Soil buffering Infographic
 gardendif.com
gardendif.com